| Fig.1 | The retina is the most important sensory area in mankind. Its general structure and organization is well known today. However, human retina also contains strange, large cell organelles which are still unfamiliar to most of us and lack a meaningful name in terminology. They were first noted by Dogiel in 1884 but mistaken as artefacts. In 1918, Kolmer was the first investigator who realised that darkly stained stripes he noted in human horizontal cell cytoplasm must be something special. He discovered strange elongate basophilic areas in the upper inner nuclear layer (Fig.2, INL) of the human retina which were lacking in almost any other species. | Fig.2
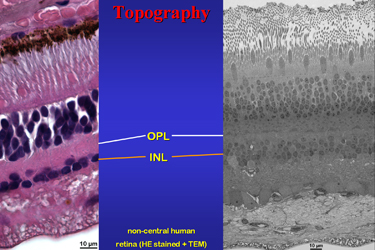 |
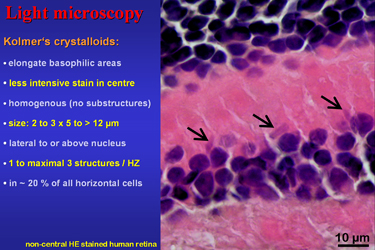
| Fig.3 | The light microscopic image in Fig.3 shows an
area with an unusual high concentration of what he called “Kristalloide”.
These elongate basophilic areas may have a less intensive stain in centre
and appear homogenous without notable substructures. Their size is about
2.5 x 5 to over 12 micrometers. They are seen lateral or above the cell
nucleus. One to maximal 3 of these structures are present in about 20%
of all horizontal cells.
Fig.4 shows some more examples of long, cigar-like cytoplasmic inclusion bodies from non central Hämatoxylin-Eosin stained human retina that was |
Fig.4
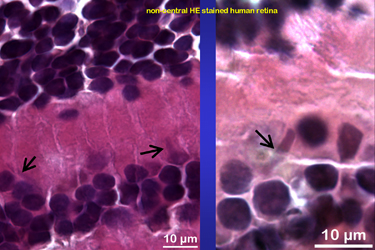 |
| Fig.5 | kindly provided by a 62 year old patient who
had to undergo an enucleation due to a malignant melanoma. The tumour-free
half of his eye was cut off and fixed as freshly as possible. Further material
from multiple organ donors was obtained from enucleated bulbs of which
the cornea was removed. Isolated non central retina was dissected in tiny
parts and further processed for light and electron microscopy.
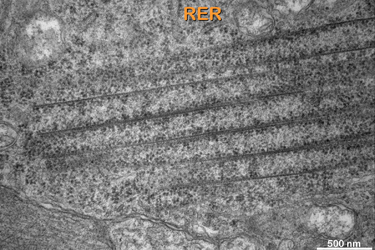
Fig.6 demonstrates a typical horizontal cell, the only location in the retina where the cell organelles appear. Here we see them cross-sectioned in the cytoplasm. We decided to call them Macrotubuli aggregati since they are large cytoplasm-filled tubes. As we will see soon their walls consist of intermediate filaments to which ribosomes are attached. |
Fig.6
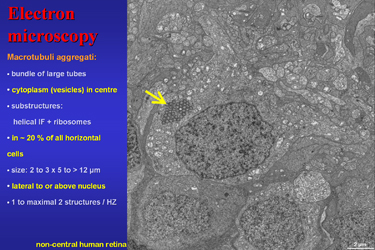 |
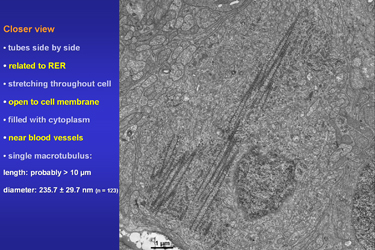
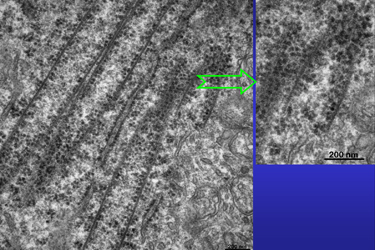
| Fig.7 | The nearly longitudinal section in Fig.7
shows an aggregate of tubes that is related to a large area of RER and
ribosomes. We may assume that some of these open macrotubuli are over 10
micrometers long. Their mean diameter is about 235 nanometers.
A closer look at the cross-section in Fig.8 reveals somewhat aggregated large tubes which often touch each other. They are filled with cytoplasm which sometimes contains vesicles of different electron density as you can see on the right. Now let us take a more detailed view at the area marked on the left. |
Fig.8
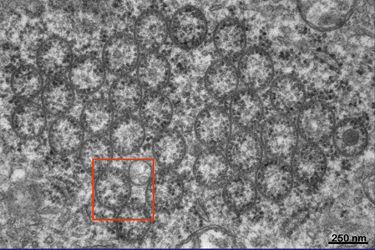 |
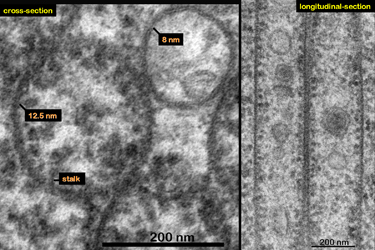
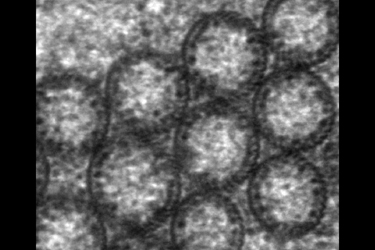
| Fig.9 | Electron dense granules, that were identified
as ribosomes by Yamada (1966), Fine and Yanoff (1972), are attached to
the inner walls of the tubes via fine stalks (Fig.9). As you may
notice the walls of these macrotubules are a little thicker than the unit
membrane. They consist of parallel running filaments that from their diameter
of 12.5 nanometers must belong to the intermediate filament group. Since
they do not run straight upward they are hardly detectable in cross- or
longitudinal sections.
Therefore we generated the animation (Fig.10) of a tilting of a thick section that demonstrates the helical arrangement of the filaments. |
Fig.10 to run the animation click here, please
 |
| Fig.11 to run the animation click here, please | First three-dimensional reconstructions also
confirm this (Fig.11).
The walls of the paralongitudinally cut macotubuli shown in Fig. 12 also show electron dense parallel running filaments. The filaments are interconnected by fine bridges over less electron dense gaps of less than 4 nanometers. |
Fig.12
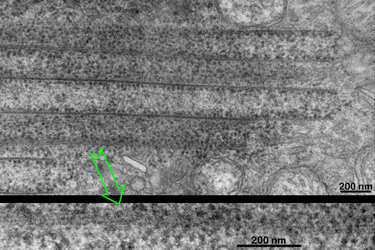 |
| Fig.13 | Tubules are cut more obliquely in this section (Fig.13). The area enlarged at the right again demonstrates the structure of their walls. In the area marked with the arrow (Fig.14) one gets the impression that a part of a tubular wall is synthesised just from the secreted proteins of the nearby open RER cistern. | Fig.14
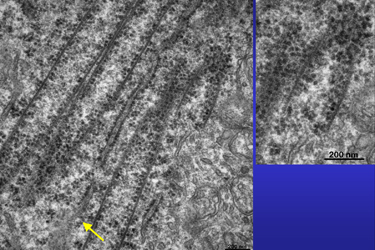 |
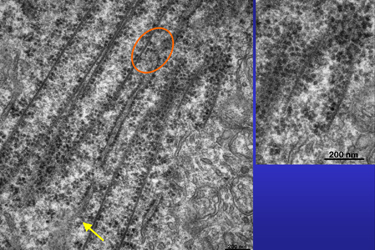
| Fig.15 | The encircled area in Fig.15 shows ribosomes
that seem to have drifted off from RER to a nearby tubular wall.
The open ends of some macrotubules are close to the cell membrane but do not seem to have any relation to it (Fig.16). Whereas the marked upper tube has a clearly defined end, the lower tube either grows or is dissolved. Note the high density of ribosomes some of which seem to be attached to filaments. |
Fig.16
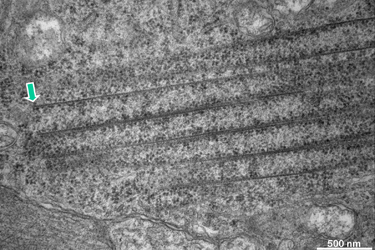 |
| Fig.17 | Note in Fig.17 some either forming or
dissolving RER which is partly bare of ribosomes in vicinity to the uppermost
tubule.
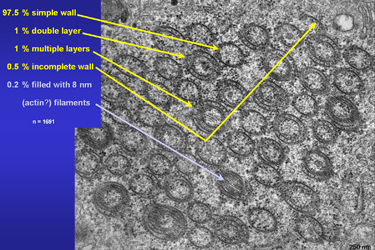
Over 97 percent of all investigated tubuli had simple, single layered walls. One percent showed a bi-layered and another one percent three to five layered walls. Fig.18 shows an unusual tube which is filled with 8 nm thick most probably actin filaments. |
Fig.18
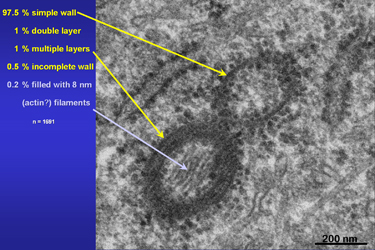 |
| Fig.19 | Fig.19 demonstrates a large group of associated
macrotubules of which many have multiple layered walls. One is entirely
filled with filaments. Further there are parts of tubular walls. We assume
that the latter aggregate directly to walls of other tubes thus forming
the multiple layers.
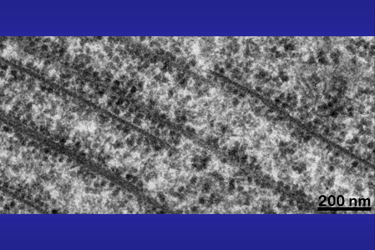
Another possibility for formation of bi- or multiple layers can be seen in Fig.20: probably two tubes failed to fuse completely at their ends in a way that partly one wall grows on top of the other. |
Fig.20
 |
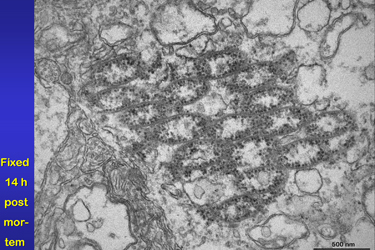
| Fig.21 | The image (Fig.21) was taken from a retina
fixed 14 hours post mortem. It demonstrates that the presented organelles
have a good stability in comparison to other nearby cell organelles like
the almost dissolved mitochondria or RER.
Though retinas of many species were investigated only very few species showed Macrotubuli aggregati (Fig.22). There are only two other locations where they were also encountered so far: 4 cases of pathological human cornea and one species where the organelles were present in the pineal gland. The animation shows a detail of tilting of a thick section with Macrotubuli aggregati possessing a double layered wall. |
Fig.22 to run the animation click here, please
 |
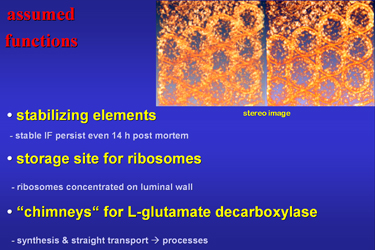
| Fig.23 | We can only speculate on the functions of these
strange cell organelles (Fig.23). At any rate by the compact arrangement
of their filaments they are stabilizing elements of the cytoskeleton. They
may serve as storage sites for ribosomes which are concentrated in their
lumen. Maybe the tubes act like chimneys and very quickly transport synthetised
L-glutamate decarboxylase to the cell processes for GABA synthesis and
required glutamate degradation.
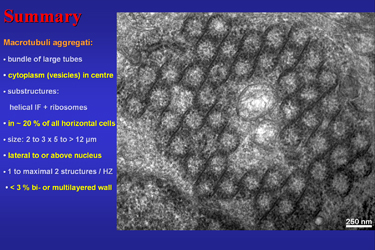
Fig. 24 demonstrates a tubuli-free area in the centre of a large aggregate of macrotubueles which is a typical finding. Further it summarises the most important findings. |
Fig. 24
 |
| Fig.25 | You can retrieve this presentation and the shown images in the electron
microscopic atlas in the internet on the linked URL: www.uni-mainz.de/FB/Medizin/Anatomie/workshop/EM/MaE.html
(Fig. 25).
Fig. 26: further investigations with antibodies will be necessary to determine the molecular nature of the filaments forming the walls of the organelles which we suggest to include as Macrotubuli aggregati into the official terminology. We thank the mentioned persons for their support and you for your attention. |
Fig. 26
 |